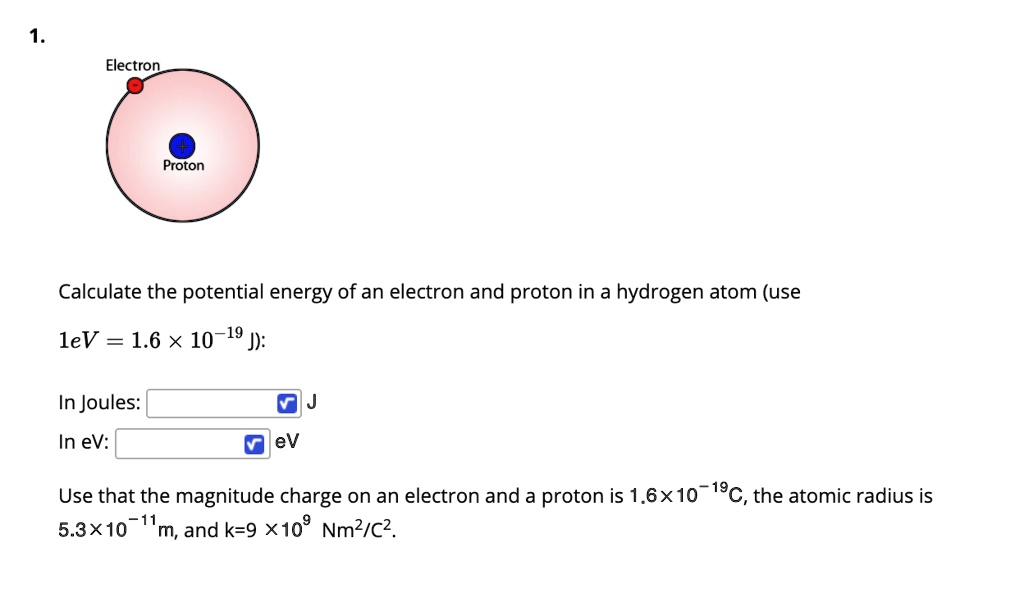
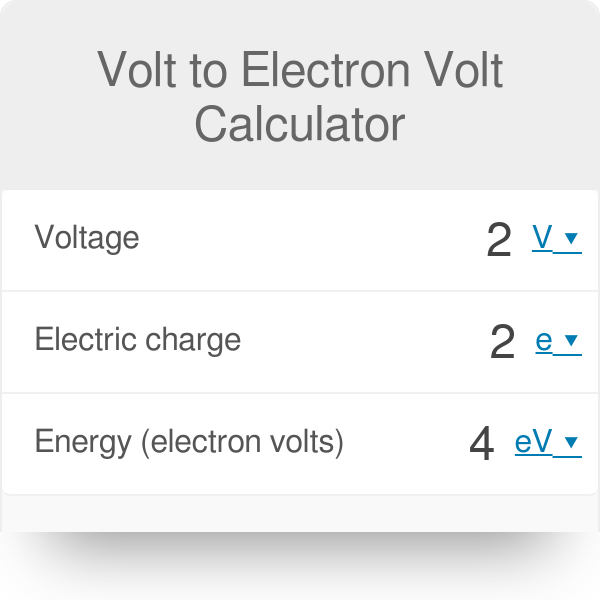
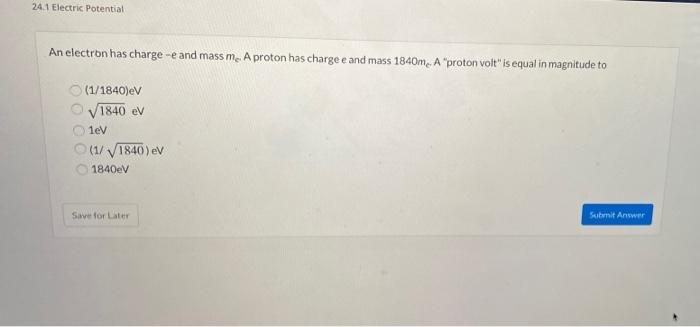
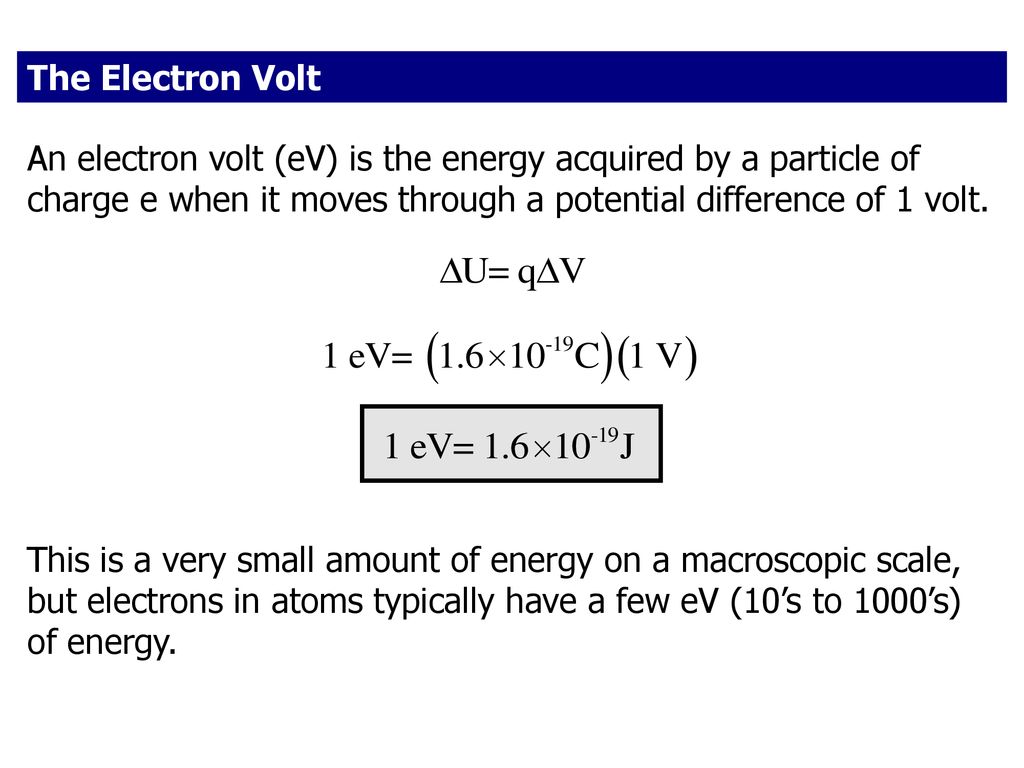
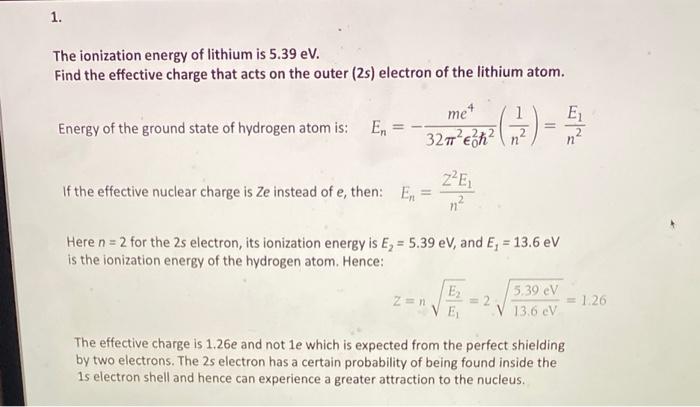
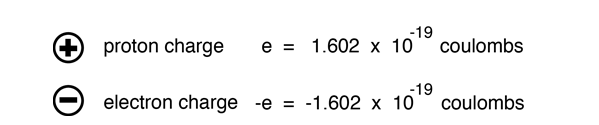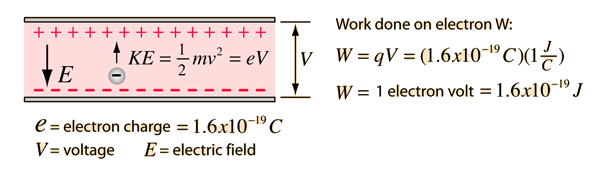The electric potential energy of electron-proton system of hydrogen atom is: (Given : The radius of electron orbit = 0.53overset {circ}{A}, electronic charge = 1.6times 10^{-19}C).

Electron Mass - Mass of electron, Charge, Speed, & value of electron 1 | Electrons, Nobel prize in physics, Engineering notes

Question Video: Calculating the Kinetic Energy of an Electron Moving through a Negative Potential Difference | Nagwa

77. An electron of 100 eV is fired directly towards a metal plate having surface charge density of -2 x 106 Cm 2. What is the distance from where the electron be
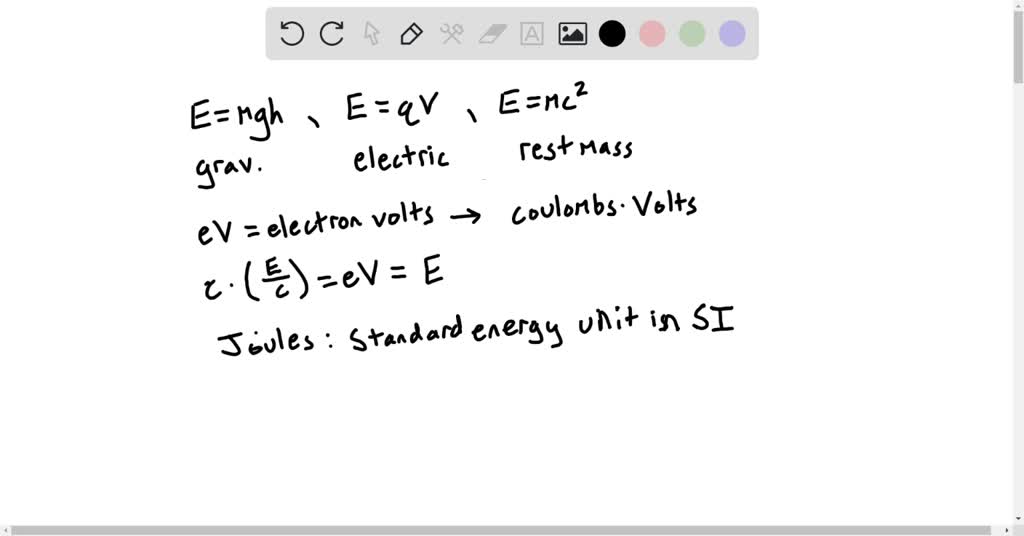
SOLVED: A common unit used to measure the energy of small particles is the electron volt (eV), which is equal to the magnitude of the charge on the electron (measured in coulombs)