Table 1 from A dispersive liquid-liquid microextraction using a switchable polarity dispersive solvent. Automated HPLC-FLD determination of ofloxacin in chicken meat. | Semantic Scholar
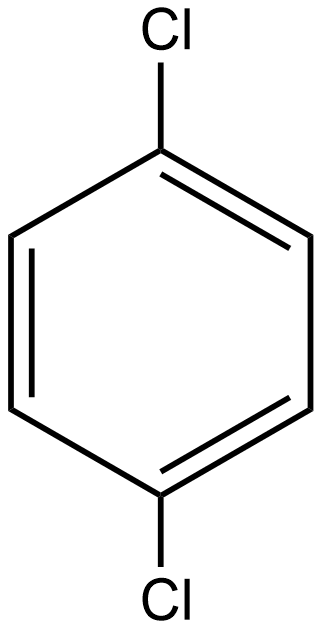
Which of the following molecule is polar:A.\n \n \n \n \n B.\n \n \n \n \n C.\n \n \n \n \n D.All of these

Explain why the dipole moment of chlorobenzene is lower than cyclohexyl chloride ? – The Unconditional Guru

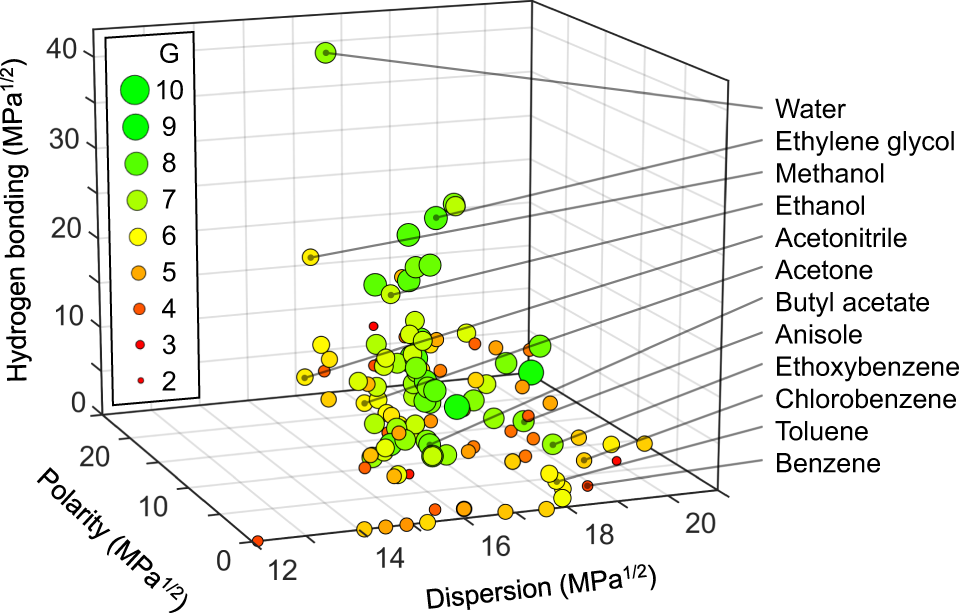
Comparison Of The Polarity Of Organic Solvents - Professional HPLC Column Hardware Consumables Supplier
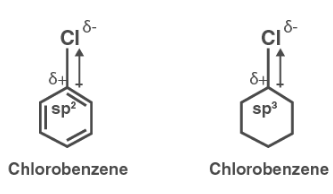
Explain why (i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride? (ii) alkyl halides, though polar, are immiscible with water? (iii) Grignard reagents should be prepared under anhydrous

Explain why the dipole moment of chlorobenzene is lower than cyclohexyl chloride ? – The Unconditional Guru

![Kannada] Why the dipole moment of chlorobenzene is lower than that of Kannada] Why the dipole moment of chlorobenzene is lower than that of](https://d10lpgp6xz60nq.cloudfront.net/physics_images/OSW_SP_CHE_XII_C10_E01_018_S01.png)


![Telugu] Explain why the dipole moment of chlorobenzene is lower than Telugu] Explain why the dipole moment of chlorobenzene is lower than](https://d10lpgp6xz60nq.cloudfront.net/physics_images/VIK_CHE_QB_C11_E02_016_S01.png)






![Punjabi] The dipole moment of chlorobenzene is lower than that of cyc Punjabi] The dipole moment of chlorobenzene is lower than that of cyc](https://static.doubtnut.com/ss/web-overlay-thumb/9968874.webp)